Contributor: Matt Anderson-Baron
NOTE: While “biomanufacturing” encompasses a wide variety of products, for the sake of brevity, this article is focused on recombinant protein based products.
The immense potential of synthetic biology
The biorevolution, decades in the making, is well underway and synthetic biology (synbio) is progressing at a blistering speed, said to be even outpacing Moore’s law (which states that computing power doubles every two years). This rapid progression in synbio is largely owed to the growing interface between biology and computers, and dramatic improvements in molecular biology tools. This has made scientists exponentially better at experimentation, rapidly increasing the rate of discovery. Many of these discoveries have fostered the development of innumerable novel biotechnologies that enable everyday products to be manufactured using biology instead of traditional methods. Today, most products like food, materials, and energy, fall outside the historical applications of biomanufacturing (i.e. medicine). However, a rapidly growing list of over 400 different products could be transitioned to production by biomanufacturing to capture a wide array of advantages.
Transitioning to biomanufacturing over traditional methods presents an opportunity to produce products more economically with enhanced properties and with a lower environmental price tag. The sweeping impact this could have on society and global economies is massive. In fact, it has been hypothesized that we could theoretically manufacture up to 60% of the inputs to our physical economy using biology.
But, if we want to make that happen, we have to address the massive chasm in global biomanufacturing capacity. Currently, we’re nowhere near equipped with the infrastructure to produce any of these novel products at a meaningful scale. And I say “meaningful” because it’s important to recognize that most of these technologies will only make a real impact if they’re deployed at a massive scale. Remember, the whole point of the biorevolution is to make food, materials, and energy better—better for humans, better for animals, but most importantly, better for the planet. But the biorevolution’s impact will be moot if its outputs are reserved for the wealthy few. To make change, these products must become ubiquitous.
Many (myself included) believe in the immense potential of synthetic biology, but we need to temper our excitement with reality by acknowledging the magnitude of this scaling challenge.
Sizing the chasm
Just how big is this chasm in biomanufacturing? The reality is, we don’t know yet. For example, if we’re going to produce 15 megatonnes of edible protein using biomanufacturing by 2030 as predicted, we’re going to need 10 billion liters of operating bioreactor capacity (the vessels in which cells are grown to produce products). Currently, there’s about 61 million liters of global bioreactor capacity. In other words, we need to increase bioreactor infrastructure by 163 times in less than a decade.That would require the production and onboarding of approximately 1,200 large scale (20,000 liter) bioreactors per week for the next 8 years straight. Note that this is only referring to edible protein (i.e. biomanufacturing applications in food). If we consider all other applications, such as material and energy, the chasm extends into the abyss.
For example, another exciting application of recombinant proteins is in materials. What’s particularly exciting is the ability to produce novel materials with enhanced properties through the rational design of proteins, such as collagens and silk proteins, by modifying their sequence (Sutherland, 2017). It has been proposed that these types of polymeric proteins could be produced recombinantly to replace petrochemical-derived polymers (Rodriguez-Cabello et al., 2009). That being said, it’s difficult to envision the scale and infrastructure requirements an application like this would demand. Currently, most recombinant proteins are produced at yields of less than 1 gram per liter. There have been reported yields in yeast as high as 22 grams per liter; however, this was far from a cost-optimized industrial process. (Hasslacher et al., 1997) Even if we assume optimistic yields (ex. 10 grams per liter), this would still require a colossal amount of infrastructure to scale this application. Regardless of the material composition (or percentage of protein content), it’s hard to imagine scaling the production of these materials to replace some volume of petrochemical-based polymers given that they are currently produced at hundreds of megatonnes per year.
To make matters more complicated, we also need to consider the speed of scaling. It’s not just a question of, “can we scale?”, but also, “can we scale fast enough”? The world’s growing demand for biologically produced goods will soon outpace our ability to supply them. The climate crisis also exacerbates our need to scale these technologies quickly; synthetic biology could be a tipping point in the fight against the climate crisis, but only if we can deploy it at scale. For reference: on average, a biomanufacturing facility for biologics takes about 3 years to build. We need rapid deployment of these technologies, and the element of speed is another challenge that cannot be answered by bioreactor-based facilities. How can we build the infrastructure fast enough?
We’re not the first to recognize the immensity of these challenges. Innovation Endeavours and KdT Ventures have already laid out the problem and discussed some potential solutions. Others have directly called out the bioreactor shortage and how it’s impeding the growth of biotech. But to date, what this discourse is missing is a thorough investigation of engineering organisms that don’t rely on steel tank bioreactors. From our perspective, that is the root of the scalability problem in biomanufacturing - its unyielding reliance on steel tanks. And circumventing that reliance with alternative organisms poses the solution to scalability.
How did we come to this abyss in the first place? Well, it’s likely because no one could have predicted this scale of demand for biomanufacturing when recombinant technology was first developed. It’s unreasonable to think that biomanufacturing systems had been designed with 10 billion liter scaling capacity in mind, when recombinant technology was first developed nearly 50 years ago. Now, as we look to break through this plateau in scale, it’s helpful to reflect on how we got here and how our dependence on bioreactors came to be.
The origins of the bioreactor status quo…
To understand today’s dependence on bioreactors, we have to reach back into history. In 1884, Theodor Escherich discovered a fast-growing strain of bacteria while studying the infant gut microbiome (Blount, 2015). This bacteria, now known as Escherichia coli (E.coli), quickly became a mainstay in the field of biology because of its key characteristics: it was easy to find and simple to work with. Fast forward some 60 years to the 1950s when the molecular biology revolution began, E.coli was cemented as the model organism to work with. Then, when recombinant technology was later developed in the 1970s, E.coli was the obvious host to work with given its prominence in the field of molecular biology and the tools that were available. By the end of the decade, the first ever licensed recombinant protein product, human insulin, was developed by Genetec and produced in E.coli (Johnson, 1983).
Following a similar timeline, the field of yeast genetics was on the rise and scientists were beginning to understand more about the biology and genetics of various species of yeast, primarily Saccharomyces cerevisiae (Fraczek et al., 2018; Roman, 1986). Following the advent of recombinant technology, yeast systems also became an obvious choice for the same reasons: they were well-understood, easy to procure, and relatively easy to manipulate. Compared to E.coli, the added advantage of yeasts was the ability to facilitate post-translational modifications (PTMs)—essential protein decorations that influence protein folding and function.
But yeast PTMs are still significantly different from mammalian PTMs, particularly for glycans (large carbohydrates that decorate proteins). Enter: Chinese Hamster Ovary (CHO) cells. CHO cells were first used in the lab in 1919. After a brief decline in their use, Theodore Puck resurrected the CHO system in 1957 and found they grew fast and had good viability, which led to their prominence as a model organism in labs around the world. Decades later, this work made CHO cells another easy choice as a recombinant protein expression system, particularly to fill the gaps that simple E.coli and yeast have. The first product produced in CHO cells, Activase, was approved in 1987. By the early 2000s, nearly 70% of the world’s human protein biologics were produced in CHO cells.
Today, these three systems, E.coli, yeast (primarily Pichia pastoris), and CHO cells produce the overwhelming majority of the world’s recombinant protein supply. Their utilization as recombinant protein factories were because of two key reasons: 1) scientists had a good understanding of their biology and genetics from previous work; and 2) they were easy to procure and work with. Now, nearly half a century later, this legacy of “good understanding and easy to work with” is the foundation for a $300 billion (and growing) bioeconomy for recombinant proteins.
But we have to ask: why did we stop there? There are a plethora of other model organisms that would be suitable for recombinant protein production that satisfy the same rubric. Why are we still using the same three organisms for most of the world’s biomanufacturing of proteins? And most importantly, why are we confining human ingenuity to the limited number of species that need to be grown in steel tanks? Why is that the box (or tank) that innovators choose to solely operate in?
What if we didn’t need steel tanks at all?
As a16z astutely points out: “It’s time to think outside of the steel tank!”
Starting from first principles
If we could start from scratch, knowing what we know today, how would we do things differently? What sort of features would make for the best biomanufacturing platform? Well, it’d be a system with:
- a highly amenable genome and the tools to engineer it;
- the ability to produce high complexity proteins;
- low CapEx and operating costs;
- simple infrastructure;
- low energy costs; and,
- massive scalability.
Any system that requires bioreactors is highly unlikely to satisfy these characteristics. To make biomanufacturing better, we need to break free from the confines of the steel tank.
There are various potential systems to replace the tank. One example is molecular farming, or the use of plants as bioreactors—an exciting platform among new and old companies alike. There are several advantages to molecular farming that circumvent many of the challenges of traditional biomanufacturing, like costly and energetic bioreactors. But it also replaces those challenges with new ones, which may be even more difficult to address. For example, most plants grow quite slowly. Many plant species currently being explored as biofactories, such as tobacco plants, require months to reach a harvestable crop. Plants also require a great deal of landmass to grow. If employed outdoors, this puts the system at the mercy of the climate (i.e. floods, droughts, etc.). If transitioned to indoor farming for better climate control, this would significantly ramp up the energetic requirements of the system. These challenges are coupled to the additional work needed downstream to process the fibrous plant material in order to extract proteins. All of that being said, molecular farming holds great potential, and the world would benefit in exploring this further as a technology. But we should also investigate more solutions that could circumvent some of these challenges.
What else is out there? At Future Fields, we looked beyond plants and sought an option that we truly felt could satisfy the above rubric and massively scale protein production. We found the answer in a tiny but mighty organism.
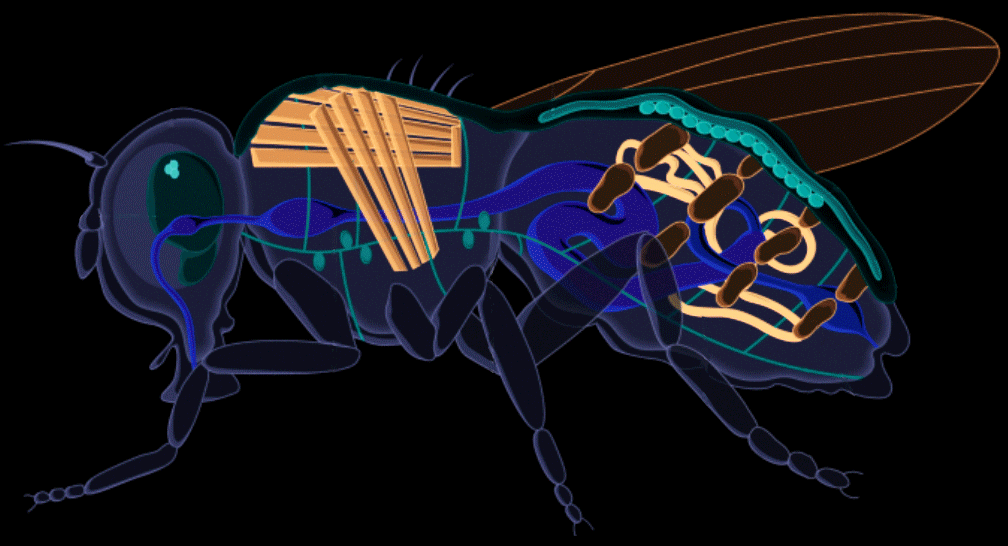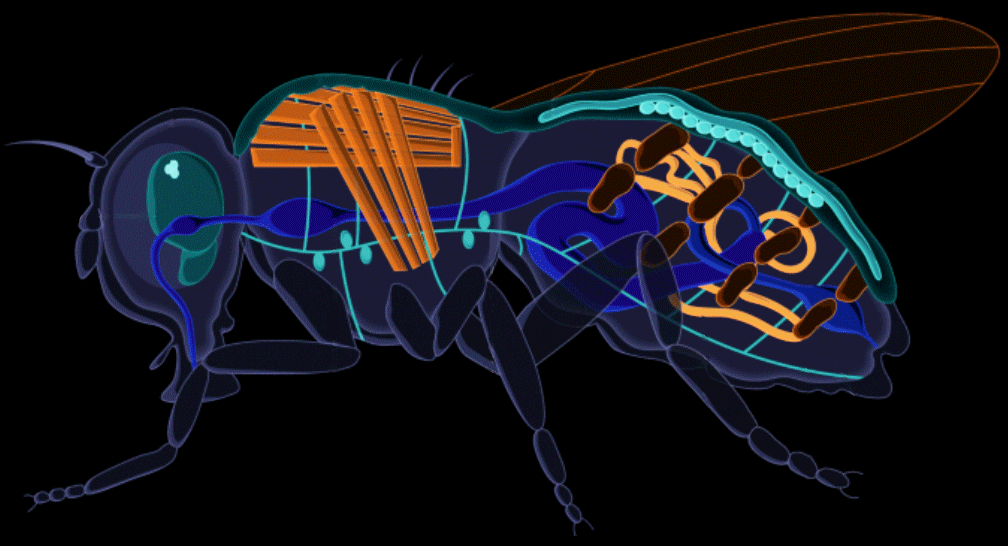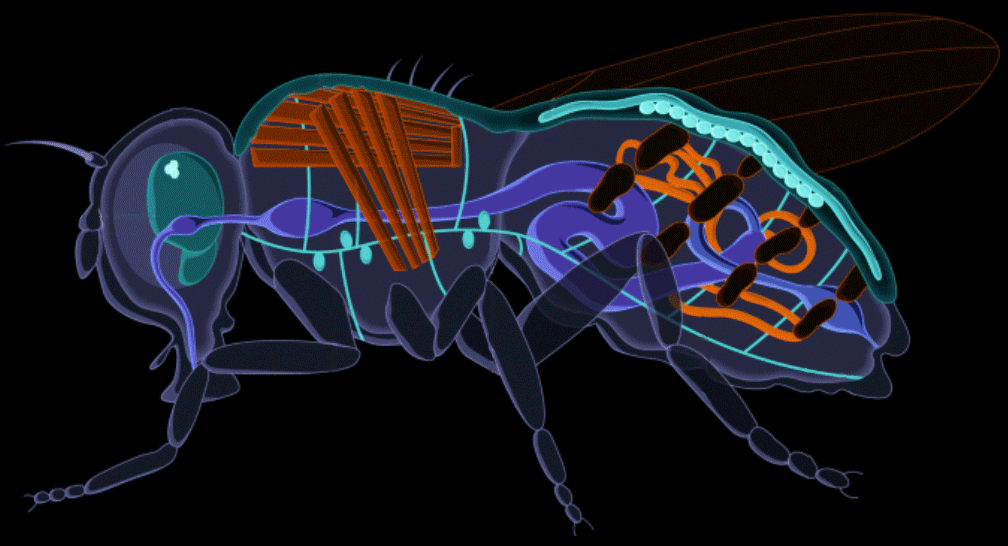
We replaced the steel tank with a fruit fly.
Drosophila melanogaster, otherwise known as the common fruit fly, has been studied for over a century for genetics and human development. Its rich scientific legacy contributing to six Nobel Prizes, fruit flies have often been described as the model organism for biological and genetic research. Harnessing this knowledge, we applied it in the recombinant protein industry to develop the ultimate biomanufacturing platform.
Stable, high-yield protein expression in fruit flies allows plateaus in cost and scalability to be broken because the system doesn't rely on complex infrastructure. It also allows for modularity in upstream production, which facilitates controlled, linear scaling, as opposed to massive step-wise jumps in scale that are typically utilized in bioreactor systems (ex. 1 liter tank to 100 liter tank to 1,000 liter tank). This offers more flexibility and control over production quantities.
Insects are some of the most efficient bio-converters in the world, which massively reduces the energetic costs of biomanufacturing. Fruit flies, in particular, have low energy requirements and develop best at near room temperature. Insects’ efficiency at converting inputs into biomass is unparalleled—there’s a reason that insects make up over half of the global terrestrial biomass. This incredible efficiency in biomass production also permits faster scaling capacity. In fact, with our production system, we can scale a given fly strain from 0 to 120 metric tons of biomass production per day in less than 60 days with minimal capex spend.
These features (low cost infrastructure, modularity, and efficient bioconversion) make insect expression systems massively scalable. Couple these features to the well-stocked genetic toolkit of Drosophila melanogaster and you have a biomanufacturing platform that can conquer the abyss that we currently face in biomanufacturing.
Conquering the abyss
Scale matters. It matters because we believe that synthetic biology is no longer a nice to have, but rather, a necessary piece of our arsenal in the fight against the climate crisis. But only if we can deploy it at scale. And we do not believe that relying solely on bioreactors will get us there.
The urgency is real. We need solutions and we need them fast. Human ingenuity has shown us the potential of using biology as a vehicle to create a more sustainable future, but in order to realize that potential, we need to go big. Again, we call on human ingenuity to create a path to scale. We need to shed our preconceived notions of what biomanufacturing is so we can realize what it could be. Conquering the biomanufacturing abyss is an immense challenge but it’s worthy of immense effort because of what’s at stake—everything. And maybe the solution to this challenge was right under our noses (and in our kitchens) the whole time—the humble fruit fly.
References
- Blount, Z.D. (2015). “The Natural History of Model Organisms: The unexhausted potential of E.coli. eLife 4:e05826.
- Johnson, I. S. (1983). "Human insulin from recombinant DNA technology". Science. 219 (4585): 632–637.
- Roman, H (1986). “The early days of yeast genetics: a personal narrative”. Ann Rev Genet. 20: 1-12.
- Fraczek MG, Naseeb S, Delneri D. (2018). “History of genome editing in yeast.” Yeast. 35(5): 361-368.
- Jayapal K. P., Wlaschin K. F., Yap M. G. S., & Hu W-S. (2007). Recombinant protein therapeutics from CHO cells — 20 years and counting. Chem. Eng. Prog. 103(7): 40–47.
- Hasslacher M, Schall M, Hayn M, Bona R, Rumbold K, Lückl J, Griengl H, Kohlwein SD, Schwab H. (1997). High-level intracellular expression of hydroxynitrile lyase from the tropical rubber tree Hevea brasiliensis in microbial hosts. Protein Expr Purif. 11(1):61-71.
- Sutherland TD, Huson MG, Rapson TD. (2017). Rational design of new materials using recombinant structural proteins: Current state and future challenges. Journal of Structural Biology. 201(1): 76-83.
- J. Carlos Rodríguez-Cabello, Laura Martín, Matilde Alonso, F. Javier Arias, Ana M. Testera. (2009) “Recombinamers” as advanced materials for the post-oil age. Polymer. 50(22): 5159-5169
*Cover art generated by DALL·E 2, enhanced by Future Fields.
More like this
Future Fields - Oct. 20, 2022